What is TILS cell therapy?
Tumor-infiltrating lymphocytes therapy (TILs) is an adoptive immune cell therapy that collects lymphocytes infiltrating around tumor cells from the cancer patient's own tumor biopsy tissue, amplifies and cultures them in vitro, and then infuses them back into the patient's body. It is a "cancer killer" for treating multiple solid tumors such as colorectal cancer, melanoma, non-small cell lung cancer, head and neck cancer, cervical cancer, and ovarian cancer! It can be used as an adjuvant treatment for early-stage cancer after surgery to eliminate residual cancer cells after surgery or radiotherapy and chemotherapy, and prevent tumor metastasis or recurrence; it can also be used as a last-line remedial treatment for advanced cancer to prolong the patient's survival time.
In fact, TIL cell therapy for colorectal cancer has long been traceable. The Annals of Oncology, a well-known journal in the field of tumors, once reported a "relationship between TIL cells and the survival of stage III colon cancer". A total of 1,532 patients were enrolled, with an average age of 58 years (range 19-86 years). The lesions of colon cancer were located on the right side (51%), the left side (48%), and unknown location (1%). The median follow-up time was 83 months. The results are as follows:
Compared with patients with low TIL levels in tumors, patients with high TIL levels had significantly longer disease-free survival (DFS). Among them, the 5-year DFS rates of right-side tumors were 57% (low TIL levels) vs 77% (high TIL levels); the 5-year DFS rates of left-side tumors were 69% (low TIL levels) vs 76% (high TIL levels) (see the figure below for details). In summary, the 5-year DFS of colorectal cancer patients is significantly correlated with TIL levels.
TILs cell therapy is significant-successfully extending the survival of patients with colorectal cancer and pancreatic cancer!
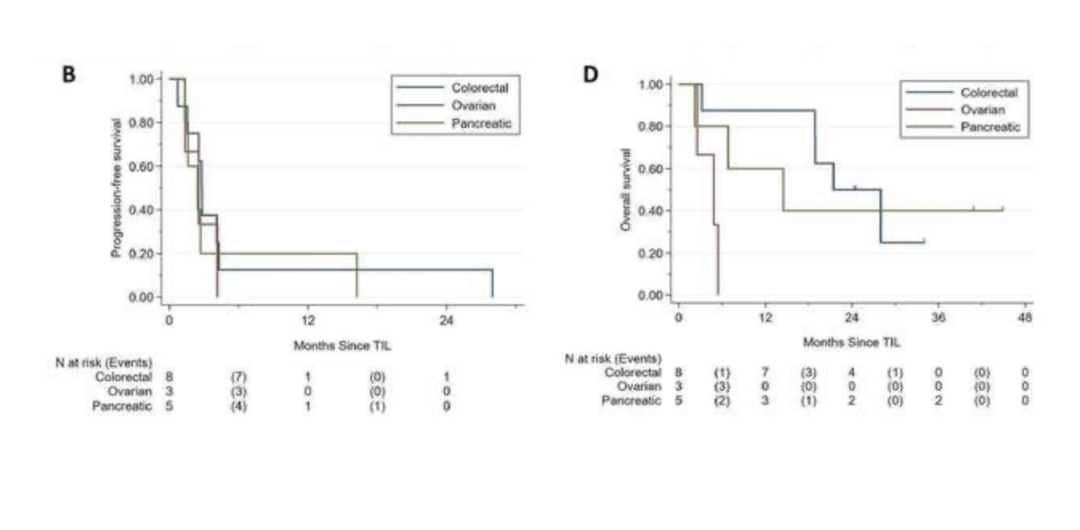
In February 2024, the Journal for ImmunoTherapy of cancer published the efficacy of tumor infiltrating lymphocytes (TILs) therapy in patients with colorectal cancer (CRC), pancreatic ductal adenocarcinoma (PDAC) and ovarian cancer (OVCA).
In this trial, 16 patients were evaluable for efficacy, including 8 patients with colorectal cancer, 5 patients with pancreatic ductal adenocarcinoma, and 3 patients with ovarian cancer. The results showed that the median survival (OS) was 18.86 months, the median survival (OS) of colorectal cancer was 21.42 months, the median survival (OS) of pancreatic cancer was 14.49 months, and the median survival (OS) of ovarian cancer was 4.86 months.
Screenshot from biosyngen official website
It is worth noting that in this trial, a pancreatic cancer patient with liver and peritoneal metastasis achieved stable disease for 17 months after TILs cell therapy, the size of the primary lesion remained stable, and the size of multiple liver metastases was significantly reduced or disappeared without any additional treatment.
TIL cell therapy BST02 is granted fast track status for the treatment of liver cancer
On February 1, Biosynthetics announced that the U.S. Food and Drug Administration (FDA) granted fast track status to the innovative TILs therapy BST02 for the treatment of all types of liver cancer, including hepatocellular carcinoma and cholangiocarcinoma.
Liver cancer is one of the most common cancers in the world. According to data from the National Cancer Center of China, the number of deaths and mortality rates from liver cancer are second only to lung cancer. Hepatocellular carcinoma (HCC) is the most common form of liver cancer, accounting for 90% of cases.
Domestic researchers announce clinical trial results of autologous TILs cell therapy in advanced liver cancer
In 2021, researchers from the Department of Hepatobiliary Surgery of Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, published the clinical trial results of autologous TILs cell therapy in advanced liver cancer in the Journal of Clinical Surgery.

Among 3 patients with advanced hepatocellular carcinoma and 1 patient with intrahepatic cholangiocarcinoma, 1 patient with liver cancer and subsequent bilateral adrenal metastasis and liver cancer and bilateral lung metastasis benefited from TILs cell therapy.
After 12 weeks of TILs cell combined with PD-1 monoclonal antibody treatment, the right adrenal metastasis was partially necrotic and alpha-fetoprotein decreased significantly.
Disease control rate 50%! Turnstone announces positive preliminary results of Phase I of TIL therapy for metastatic colorectal cancer
Colorectal cancer CRC is one of the most common malignancies worldwide, with its incidence and mortality ranking third and second among major malignancies, respectively. About half of patients are already in the advanced stage when diagnosed, and its treatment has always faced great challenges. The liver is the main organ of CRC metastasis. Metastatic advanced CRC is complex and difficult to treat, with very limited drugs available for treatment and a response rate of less than 5%.
TIDAL-01 is a next-generation tumor-infiltrating lymphocyte (TIL) therapy. By identifying, selecting and amplifying the most effective subsets of patient-specific tumor-reactive T cells, TIDAL-01 aims to improve and expand the clinical efficacy of TIL and overcome the limitations of current TIL-based therapies.
In Phase I clinical trials, TIDAL-01 showed good tolerability in terms of safety, and no serious adverse events were observed. Preliminary evaluation results showed that among the four evaluable patients, the overall response rate (ORR) was 25%, the disease control rate (DCR) was 50%, and one patient showed a durable complete remission (CR). In terms of efficacy, 50% of patients showed sustained clinical benefits, and the progression-free survival of patients with sustained complete remission exceeded one year, while the progression-free survival of another patient with stable disease was 6 months.
New TILs therapy-miraculous complete remission in patients with advanced colorectal cancer
Colorectal cancer (CRC) is the third most common cancer, with a five-year survival rate of about 65%, and a five-year survival rate of less than 20% for metastatic patients. About 85% of patients are microsatellite stable (MSS), and about 15% of patients are microsatellite unstable (MSI). Among them, MSS tumors are generally considered "cold" tumors, which respond poorly to immunotherapy, and anti-PD-1 or PD-L1 therapy has little effect. For patients who lack actionable mutations or who have failed targeted therapy, the median overall survival (mOS) is 6.4 to 10.8 months; the median progression-free survival (mPFS) is only 2.0 to 5.6 months.
TIDAL-01, a new TILs therapy developed by Turnstone Biologics in the United States, cuts fresh tumors into pieces or cultures them free in primary expansion (preep). Antigen presenting cells (apcs) are isolated and amplified from the patient's matched blood. Peptides encoding mutations are synthesized, loaded onto apcs, and co-cultured with autologous TILs. Compared with traditional TILs cells, after co-culturing TILs and peptide-loaded APCs, the reactivity of new antigens in FACS is significantly enhanced, and the anti-cancer lethality is doubled! The disease control rate (DCR) reached 50%, the overall response rate (ORR) reached 25%, 50% of patients showed sustained clinical benefits, and the progression-free survival of patients with sustained complete responses exceeded 1 year. One of the patients was lucky enough to achieve a deep and lasting complete response, that is, complete remission (CR)!
END
Since its first clinical application in 1988, TIL therapy has gone through more than 30 years of development. Compared with other T cell therapies, TIL therapy has the magical power of "turning waste into treasure" for the patient's own tumor tissue. It has unique advantages in the treatment of solid tumors and can even help some solid tumor patients achieve long-term survival with tumors! In recent years, the field of TIL research and development has made breakthrough progress. Since August alone, many innovative TILs therapies have been launched in China and the United States.
my country's TILs therapy is based on the tumor infiltrating lymphocyte (TIL) therapy in the United States, and has been specially improved to increase the self-amplification ability of TILs cells and overcome the tumor microenvironment. The good news is that currently, many TILs therapies in my country have been officially approved for clinical research, mainly targeting non-small cell lung cancer, esophageal cancer, bile duct cancer, breast cancer, ovarian cancer, cervical cancer and other solid tumors.






