Allow normal cells to continue to divide and proliferate;
Evaluate damaged cells. If the degree of damage is not serious, it can assist cells in repair;
For abnormal cells with severe damage or chromosome structural mutations, the TP53 gene will inhibit cell division and guide cells to undergo apoptosis. By causing these bad cells to commit suicide, they can prevent them from continuing to divide and prevent tumors from occurring.
Upregulate multiple anti-cancer genes and downregulate the activity of multiple oncogenes.
Help cells cope with oxidative damage and further protect cell DNA.
Activate and repair damaged DNA to prevent cell canceration and maintain genome stability.
The P53 tumor suppressor gene can regulate cell growth in the S phase and G1 phase to prevent cell canceration.
It can specifically cause programmed cell death of tumor cells or put tumor cells in a severe hibernation state without damaging normal cells.
High-risk groups prone to tumors;
Middle-aged and above, long-term living and working environment pressure;
Long-term exposure to harmful chemicals and radiation;
Long-term smokers and drinkers, and those who consume too much high-fat food and become obese;
Physical examination found abnormal tumor markers;
Suspected tumors were found in the body;
People with a family history of cancer or genetic susceptibility.
Breast fibrocystic disease;
Chronic gastritis and intestinal metaplasia;
Chronic ulcerative colitis;
Colorectal adenomatous polyps;
Mucosal leukoplakia;
Chronic inflammation: such as EB virus, HBV hepatitis B virus, HCV hepatitis C virus, HPV human papillomavirus infection, HP Helicobacter pylori infection, esophageal reflux, chronic lung inflammation, prostatitis, etc.
For people with multiple malignant solid tumors (liver, lung, digestive tract, breast, cervix, etc.) combined with radiotherapy, chemotherapy, hyperthermia, and immunotherapy.
Abnormal tumor markers in patients in the recovery period.
P53 gene therapy

What is the P53 gene?
The recombinant human P53 adenovirus injection was named Gendicine/今又生 by Vice President Zeng Qinghong. It is a recombinant replication-deficient human adenovirus type 5 carrying the wild-type p53 gene developed by Shenzhen SaiBianuo Company. It can stimulate the body to produce specific anti-tumor immune responses by expressing the tumor suppressor gene p53; the drug is an intratumor injection and was approved for marketing in China in 2003. It is mainly used to treat head and neck squamous cell carcinoma and is the world's first anti-tumor gene therapy product. It is also named because it encodes a protein with a molecular weight of 53kDa. It is a widely studied tumor suppressor gene. One of its important functions is to regulate cell division and proliferation. It is the most common mutated gene in human cancer. More than 70% of cancers have P53 gene mutations. Under normal circumstances, the TP53 gene monitors the orderly division of cells.
The role of recombinant human P53 adenovirus injection
Recombinant human P53 adenovirus injection is the world's first gene drug: the world's first marketed tumor gene therapy drug for the prevention/treatment of solid tumors in the field of genes, creating a new era of human gene therapy for diseases.

In February 2018, the international medical journal "Human Gene Journal" published a 12-year clinical review of the treatment of recombinant human P53 adenovirus injection, summarizing the safety and effectiveness of clinical treatment since the launch of "recombinant human P53 adenovirus injection".
In November 2021, the "Expert Consensus on the Treatment of Head and Neck Tumors with Recombinant Human P53 Adenovirus Injection" was published in the "International Journal of Oral Science". All Phase I-IV clinical trials have been completed and passed the national review for safety and reliability, with almost no adverse reactions.
Indications
Tumor prevention
Precancerous lesions
Cancer treatment and recovery period
P53 Patent Certificate
Patent Name | Country | Patent Number | Content Covered |
A method for producing recombinant adenovirus | China | ZL98123346.5 | Production process invention |
Recombinant of viral vector and human tumor suppressor gene and its application | China | ZL02115228.4 | Product invention, product structure, clinical application, etc. |
Australia | AU2004217830 | ||
Turkey | TR200503561B | ||
Russia | RU2005127813 | ||
Canada | CA2518169 | ||
Genetic recombinant drug of adenovirus vector and p53 gene for treating proliferative diseases | China | ZL03125129.3 | Mechanism of action, clinical application, etc. |
Australia | AU2004240968 | ||
South Korea | KO0880701 | ||
Europe | EP04731832.4 | ||
Japan | JP4695086 | ||
New use of recombinant adenovirus p53 products in tumor treatment | China | ZL200510002779.1 | Resistance to toxic and side effects associated with chemotherapy and radiotherapy, and improving the quality of life of patients |
Australia | AU2005326331 | ||
Europe | EP1842921 | ||
Russia | RU2007128308 | ||
Peptide-liposome and human vascular endothelial growth factor gene recombinant plasmid complex and use | China | ZL02134321.7 | Product invention, product structure, clinical application, etc. |
Human embryonic kidney 293 cell subclone cell line | China | ZL03126889.7 | Engineered cells |
Activated Bax gene for the treatment of malignant tumors using adenovirus as a vector | China | Published, application number: 201110089525.3 | AdBax Product structure, construction method and use in treating tumors |
New application of P53 gene | China | CN102895676 | Treatment of type II diabetes |




P53 drug invention

P53 drug qualification

Drug production license (December 2021)
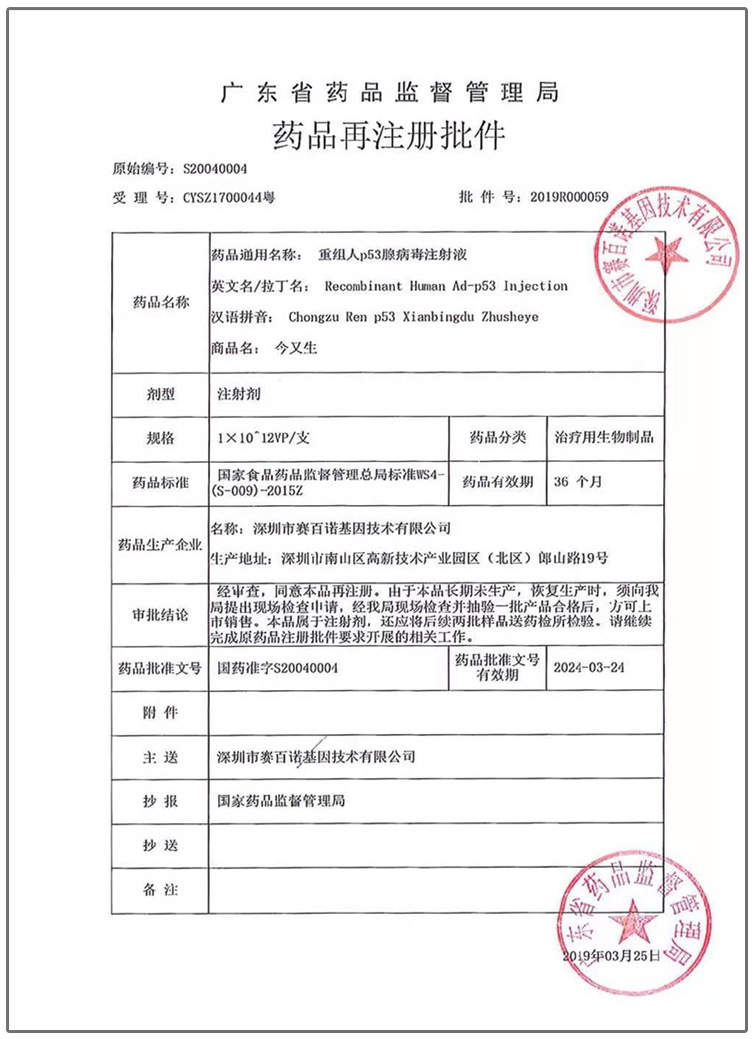
Drug re-registration approval (September 2019)
What are the advantages of P53 compared with other "anti-cancer injection" products?
Recombinant human P53 adenovirus injection | Other "anti-cancer injections" | |
Legality | Nationally approved for marketing of a class of new biological drugs With drug production license (available on the official website of the Food and Drug Administration) | None |
Safety | Safe and reliable after national approval I—ⅣPhase I-IV clinical trials have been completed Almost no adverse reactions | Unknown Missing clinical trials There are certain unknown risks |
Effectiveness | Clinical (CR+PR) as high as 90%--96% | Unknown |
Broad spectrum | All solid tumors can be prevented/treated | Few types of tumors can be prevented and treated |
Uniqueness | The only drug on the market in the field of genetics to prevent/treat solid tumors in the world We are the only one in the country and even the world that can use this drug | It can also be used in China/abroad |
Scarcity | Annual production is limited and supply is tight | As long as there is money, there is goods |
P53 success stories
- Next
- None






