TILs stands for Tumor Infiltrating Lymphocytes. TILs are immune cells found in tumor tissues. Unlike other similar T and NK lymphocytes circulating in the blood, TILs are a type of lymphocytes that penetrate deep into the interstitial tissue of tumors and have stronger killing ability. The main types include T cells and NK cells.
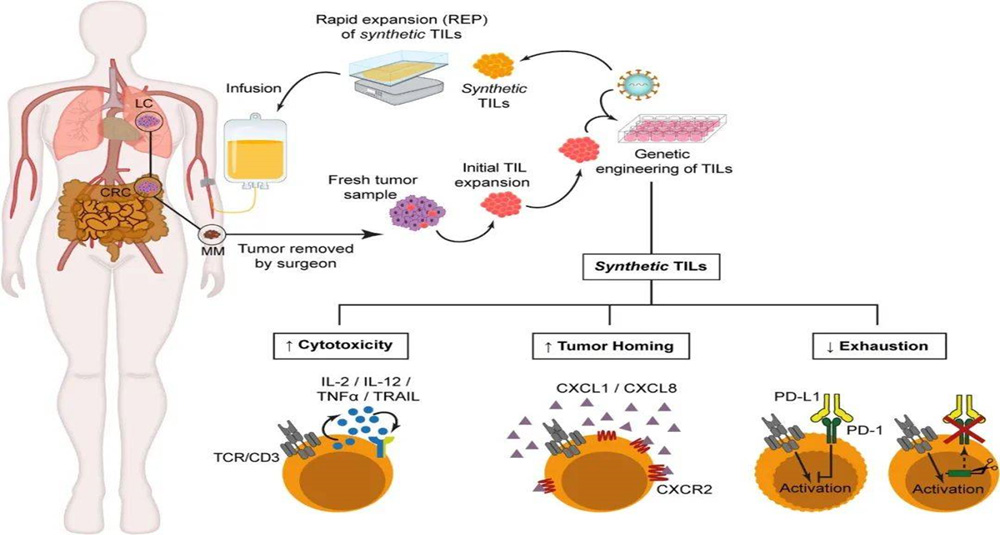
TIL therapy is a method of separating T lymphocytes from tumor tissues, stimulating and amplifying them in vitro, and then returning them to the patient's body. It can treat primary or secondary tumors by inducing cellular immune responses, expanding immune responses, and releasing cytotoxins to identify and kill tumor tissues.
Derived from the patient's own tumor tissue, it has strong specificity and is a precise individualized treatment;
Rich tumor-specific targets can achieve a broad spectrum of cancer cell killing effects;
Good tumor tropism, with super tumor homing and infiltration ability;
High safety, low toxicity and side effects, and less impact on normal tissues;
Can have long-term anti-tumor memory and provide lasting anti-tumor protection;
The number of in vitro amplification is large, reaching tens of billions to hundreds of billions of times.
Extract lymphocytes
Extract infiltrating lymphocytes (TILs) from the patient's own tumor tissue.
In vitro expansion
The extracted TILs are expanded under special culture conditions to make these cells proliferate in large quantities in vitro.
Selection and optimization
The expanded TILs are screened and optimized to improve their ability to identify and attack tumor cells.
Reinfusion to patients
The expanded and optimized TILs are returned to the patient by intravenous injection.
Combination therapy
TILs therapy is often used in combination with other treatments (such as chemotherapy, radiotherapy, or immune checkpoint inhibitors) to improve efficacy.
TILs' immune cells come from tumor tissue, while other cell immunotherapies mostly come from blood.
Use tumor mutation information to find lymphocytes that can most effectively target and accurately identify mutated cancer cells.
After separation and screening, TILs cells are amplified to the maximum extent, and then re-injected into the patient's body after ensuring the effectiveness and activity of T cells, greatly improving the body's anti-cancer "troops".
TILs cells simultaneously demonstrate three major functions: tumor recognition, overcoming tumor microenvironmental barriers, and efficient amplification, achieving a strong anti-cancer effect.
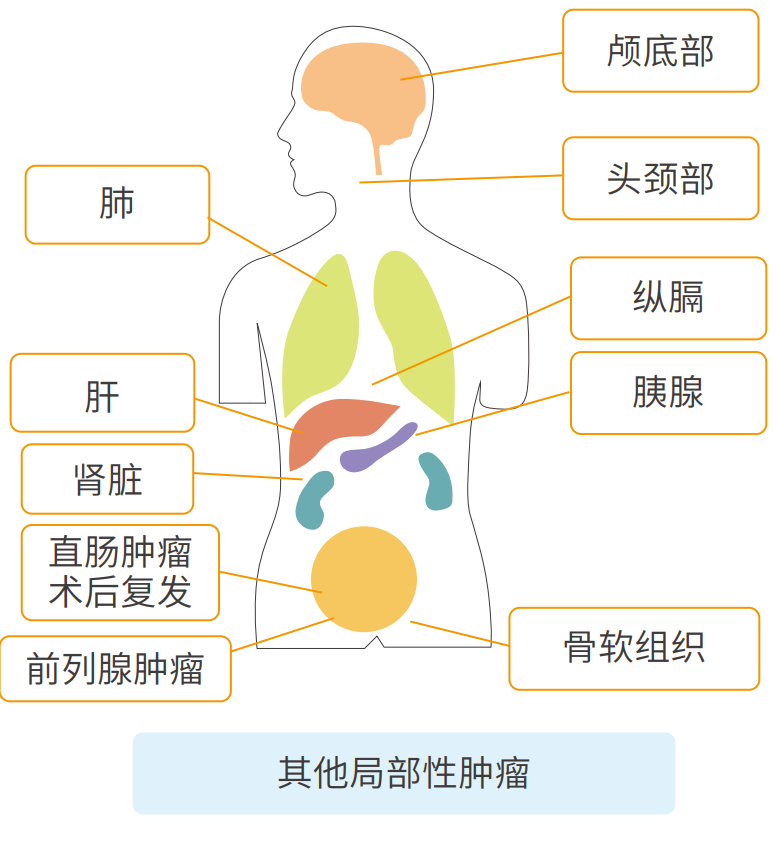
Head and neck tumors (otolaryngology, oral and facial surgery, etc.);
Tumors at the base of the skull (meningioma, chordoma);
Esophageal tumors, used in combination with chemotherapy;
Mediastinal tumors (thymic tumors, posterior mediastinal tumors);
Lung tumors;
Liver tumors;
Bile duct tumors (used in combination with chemotherapy);
Kidney tumors;
Unresectable locally progressive pancreatic tumors;
Prostate tumors;
Soft tissue tumors (malignant);
Local recurrence of rectal tumors after surgery;
Uterine cervical tumors, uterine body tumors;
Metastatic tumors (the number of lung and liver lesions is less than 3).
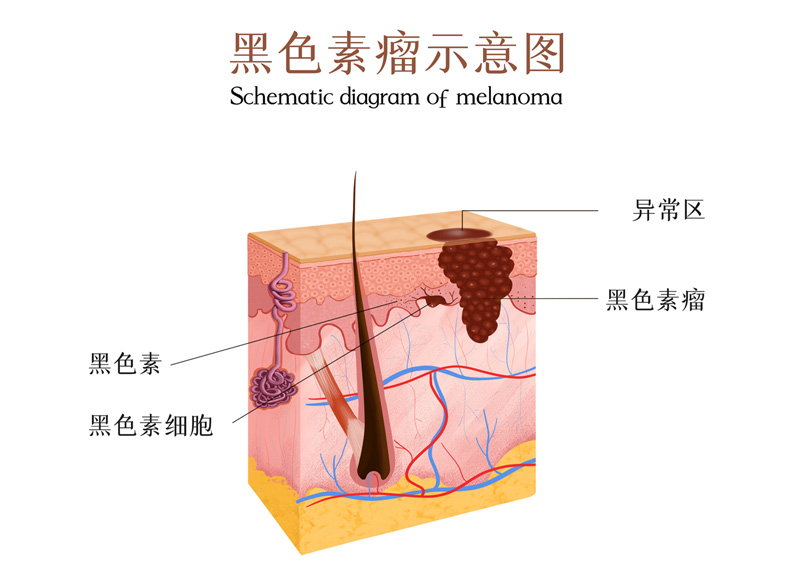
Melanoma
Non-small cell lung cancer
Advanced recurrent/metastatic breast cancer
Advanced recurrent/metastatic cervical cancer/ovarian cancer
Gastric cancer, colorectal cancer
Advanced liver cancer
Prostate cancer
Head and neck cancer
Choledochocarcinoma, gallbladder cancer
Anaplastic thyroid cancer
Malignant tumors such as sarcoma

What are TILs and TIL therapy?
Advantages of TILs therapy
Key steps of tumor-infiltrating lymphocytes
How TILs can accurately kill cancer cells
Rising star in solid tumor treatment
Which cancer patients are suitable?
TILs therapy has been tested in a large number of clinical trials for solid tumors and has shown excellent therapeutic potential, including:
Typical case of melanoma treatment
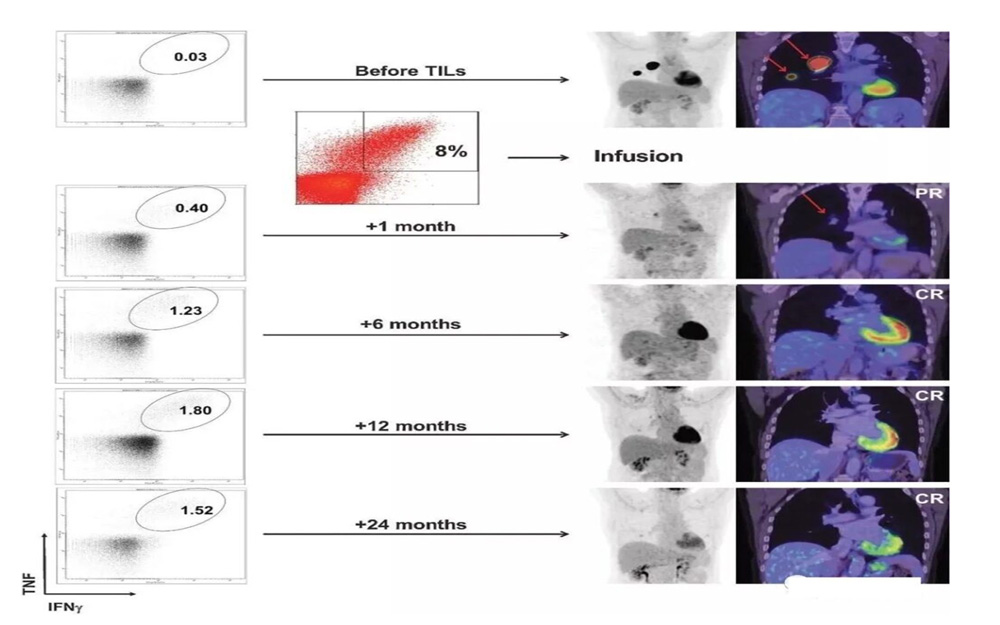
A patient with advanced melanoma had extensive tumor metastasis before treatment. After 1 month of TILs treatment, the lesions were significantly reduced and complete remission was achieved within 6 months after treatment. After two years of treatment, it is still in complete remission, and tumor-reactive CD8+ T cells persist.
TILs therapy can produce durable responses in 144 patients with advanced melanoma, showing a positive objective response rate: the disease control rate (DCR) is as high as 80.3%; the objective response rate (ORR) reaches 36.4%; (complete response reaches 4.5%, partial response is 31.8%), and 43.9% of patients have stable disease.
Combined with vemurafenib treatment of metastatic melanoma case
After a patient received an initial 9.27×10^10 treatment of vemurafenib, TIL and 5 doses of IL-2, the patient's multiple subcutaneous masses on the left thigh were significantly reduced. After TILs treatment, the metastasis continued to shrink and completely disappeared within 12 months (Figure 1B)
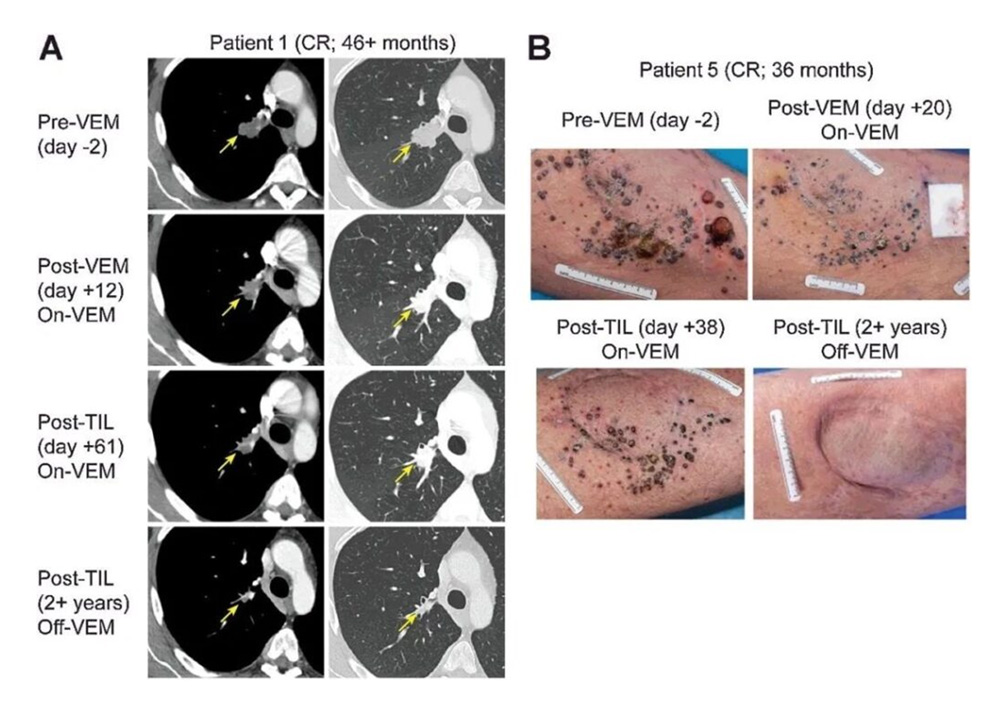
The patient with the best treatment effect: a 46-year-old male with BRAF V600E mutation-positive melanoma, accompanied by lymph node metastasis, liver metastasis, and left adrenal enlargement.
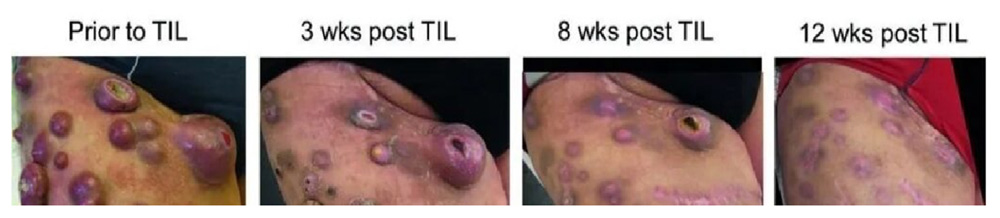
During the treatment with vemurafenib, the patient's liver metastasis completely disappeared, but lymph node metastasis progressed. He was then treated with ipilimumab, but the disease progressed after 4 courses. At the time of entering the study, the patient had extensive metastasis to the right thigh, as well as lymph node metastasis and adrenal enlargement, but no signs of liver, lung or brain metastasis. Afterwards, the patient received 19.6×10^10 TILs, followed by 4 high-dose IL-2s, and achieved complete remission 12 weeks after TILs infusion.
Typical case of colorectal cancer treatment
140 billion immune cells enter the body and defeat 7 tumor lesions: In 2013, Celine Ryan unfortunately suffered from colon cancer. After receiving radiotherapy and chemotherapy, the cancer continued to recur, cancer cells metastasized to the lungs, and 7 tumors grew. In desperation, Celine participated in a clinical trial of the National Cancer Institute of the United States. The clinical trial was led by Professor Steven Rosenberg, a world-renowned oncologist.
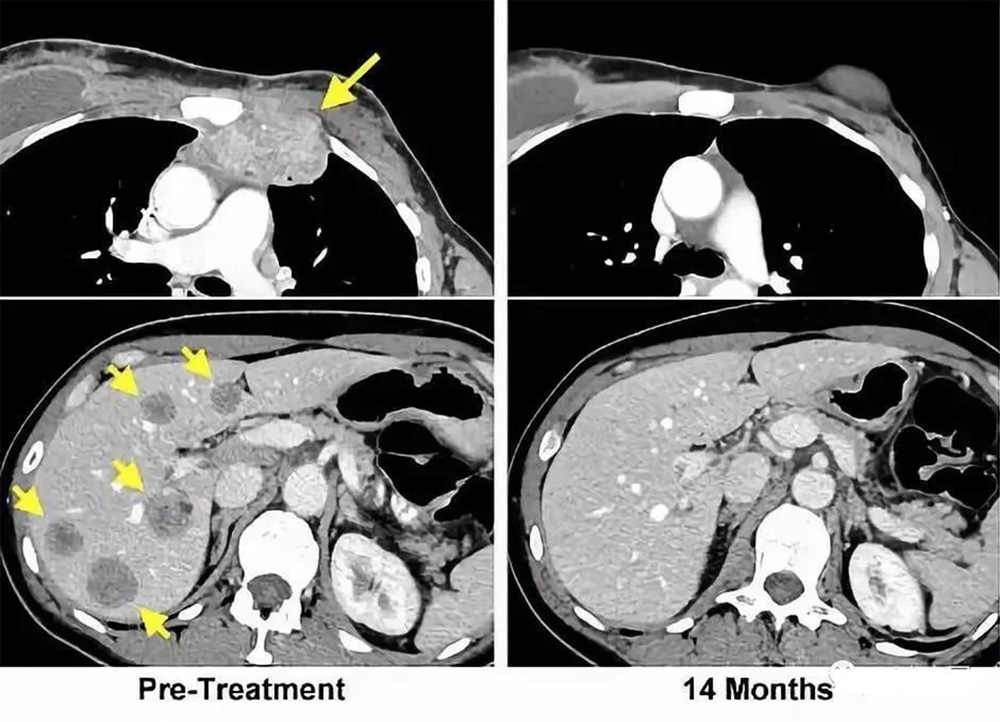
The yellow arrow on the left is the location of the tumor before treatment; the picture on the right is a review 14 months after the end of treatment: the tumor has completely disappeared.






