Adenovirus particles are more stable
Better safety and easier to operate
Adenovirus genome has greater potential
Adenovirus genome is more efficient
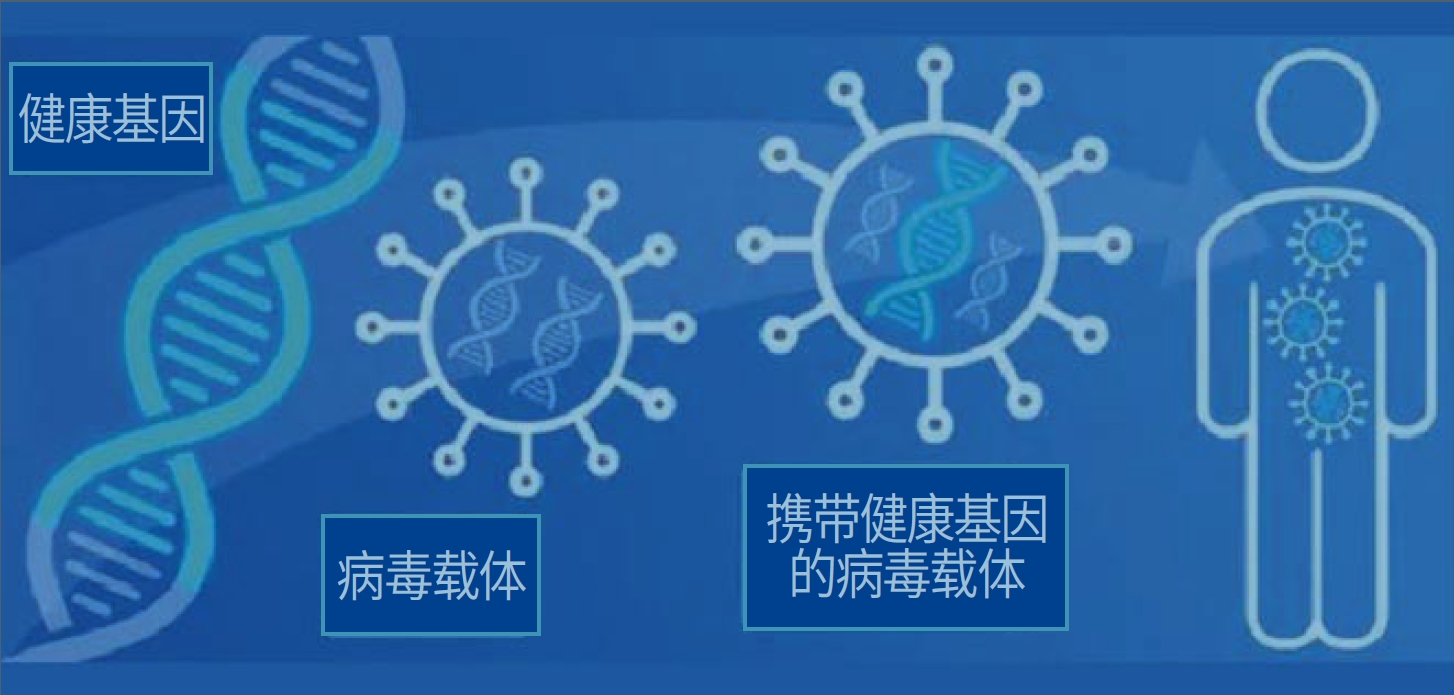
The second generation of adenovirus type 5 is used as a carrier to carry human endostatin gene
ntratumoral injection, in vivo expression of human endostatin protein
Inhibit tumor angiogenesis, block the blood supply of tumor tissue, induce tumor cell apoptosis, induce tumor immunity, and turn "cold" tumors into "hot" tumors
Treatment methods with almost no drug resistance
Short time, no need for hospitalization
No side effects, no harm to normal cells
Combined with other therapies, the effect is significant
No age limit, can act on all cancers
Targeted therapy
Highly targeted tumor blood vessels
Gene therapy
Sustained and efficient, long-term tumor suppression
Low dose, high efficiency
Little systemic toxicity and side effects, good patient tolerance
Target effect
Improve comprehensive treatment and reduce drug resistance
Combined with other therapies
Prevents tumor metastasis
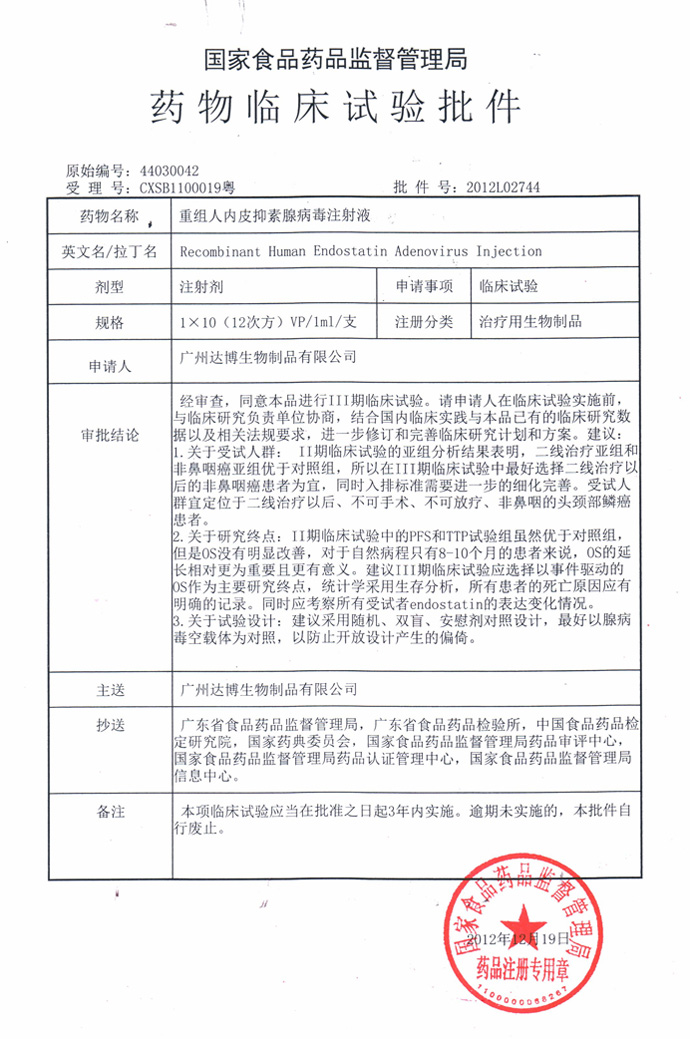
E10A has obtained the drug clinical trial approval from the State Food and Drug Administration
Approval number: 2012 L02744
E10A received the inspection report from the China Food and Drug Inspection Institute on December 28, 2021
Inspection number: SH0414202105913
E10A received the drug clinical trial approval notice from the State Drug Administration on April 7, 2023
Notice number: 2023LP00603
Prepare the recombinant human endostatin adenovirus injection according to the regulations;
After disinfection and anesthesia, inject E10A into the tumor, and multiple angles and multiple points can be injected into the tumor.
Injection dose: 1.0×1012 VP。
What is E10A anti-tumor therapy
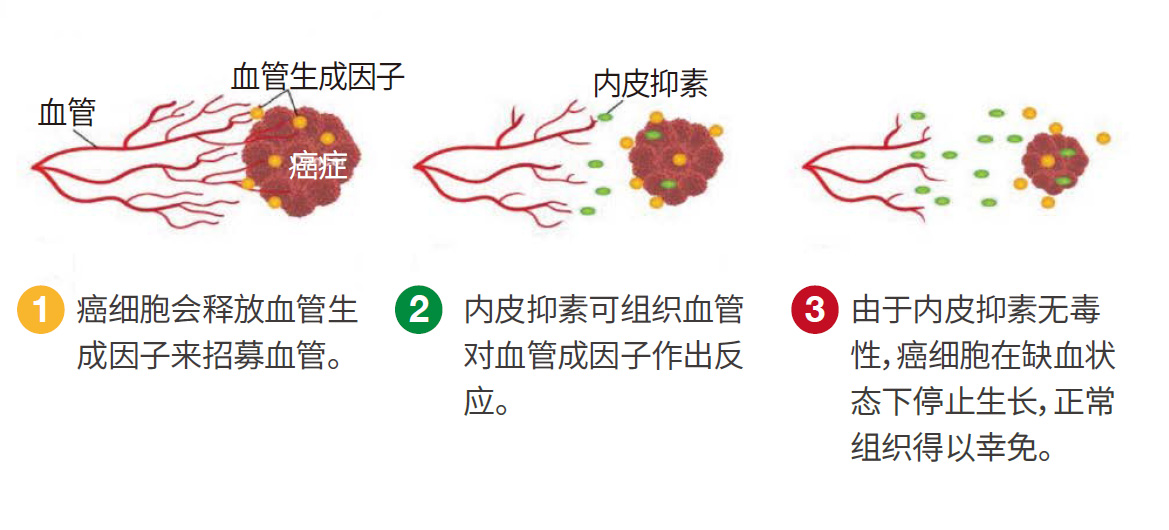
E10A is called Recombinant Human Endostatin Adenovirus injection. It is an anti-tumor preparation made by genetically recombining human endostatin gene and type 5 adenovirus particles. After intratumoral injection, it can efficiently express human endostatin protein in the local tumor tissue, specifically inhibit the proliferation and migration of tumor vascular endothelial cells, induce apoptosis of tumor vascular endothelial cells, inhibit tumor angiogenesis, block the oxygen and nutrient supply of the tumor, and make it "starved to death", so that the tumor cells die naturally or necrotize, achieving an anti-tumor treatment method to inhibit tumor growth and metastasis.
Anti-tumor principle of E10A
Gene drugs with adenovirus as carrier have the following advantages:
Advantages of gene therapy
Basic structure of E10A · Advantages of anti-tumor mechanism
Main developer of E10A

Academician Huang Wenlin
Academician of the European Academy of Natural Sciences, professor of Sun Yat-sen University Cancer Hospital; selected expert in national key fields, enjoys special allowances from the State Council; 973 infectious disease expert group (12th Five-Year Plan) expert; rotating chairman of the Asia-Pacific Cell and Gene Therapy Association; deputy chairman of the Gene Therapy Branch of the China Pharmaceutical Biotechnology Association; Honorary Professor of the Chinese University of Hong Kong;
He presided over the national "11th Five-Year Plan to 13th Five-Year Plan" major new drug creation project, "863", "973" projects and National Natural Science Foundation and many other projects. It has obtained more than 30 invention patents in the United States, international PCT and China, and has won the 12th China Invention Patent Gold Award, the Second Prize of the National Science and Technology Progress Award, the First Prize of the Ministry of Education Natural Science Award, and the First Prize of Guangdong Science and Technology Progress Award. It has developed 5 innovative gene therapy and immunotherapy products for tumors and metabolic diseases, and developed China's first gene therapy drug to complete Phase III clinical trials (recombinant human endostatin adenovirus injection) and successfully transformed it into a product needed by the market.
About the patent certificate of E10A

About the clinical trial approval of E10A
Indications
For the treatment of solid tumors such as head and neck squamous cell carcinoma
Injection administration SOP
[Selection of injection lesions]
Select one or more injection lesions for intratumoral injection, and avoid lesions with skin infiltration or cancerous ulcers.
According to the results of imaging examination (CT/MR: head, neck, chest, upper abdomen, lower abdomen and pelvis), record up to 5 evaluable target lesions according to the RECIST1.1 evaluation criteria, and select the most suitable lesion with the largest volume as the main lesion for intratumoral administration. If multiple lesions of similar volume (≥80% of the largest lesion volume) appear, inject the test drug into each lesion according to the lesion volume ratio, and the total amount remains unchanged. Before injection, the ultrasound doctor will evaluate the injection risk of the target lesion to avoid selecting lesions with high intratumoral injection risks.
[Basic process]
The route of administration in this study is intratumoral injection, and the operation is as follows:
Phase II clinical study-case
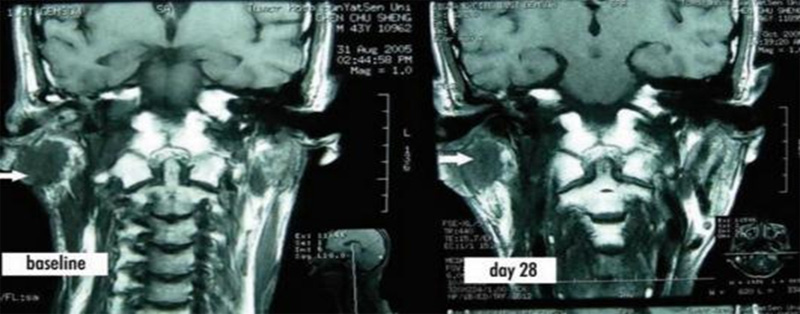
Case 1: Metastatic nasopharyngeal carcinoma in the right posterior nasopharynx. The size of the nasopharyngeal mass was 3cm×2.5cm×2cm at baseline, and it shrank to 2cm×1.5cm×1cm on days 21 and 28.
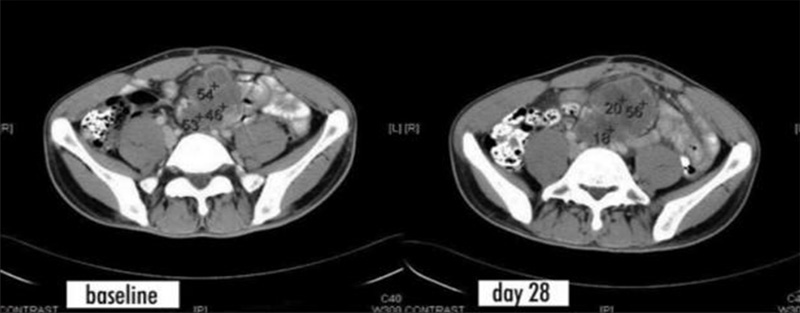
Case 2: The patient had metastatic colon cancer. The CT scan on day 28 showed irregular areas with lower density, and the CT value decreased from 46-54 before treatment to 18-20, indicating that the tumor was necrotic after treatment.
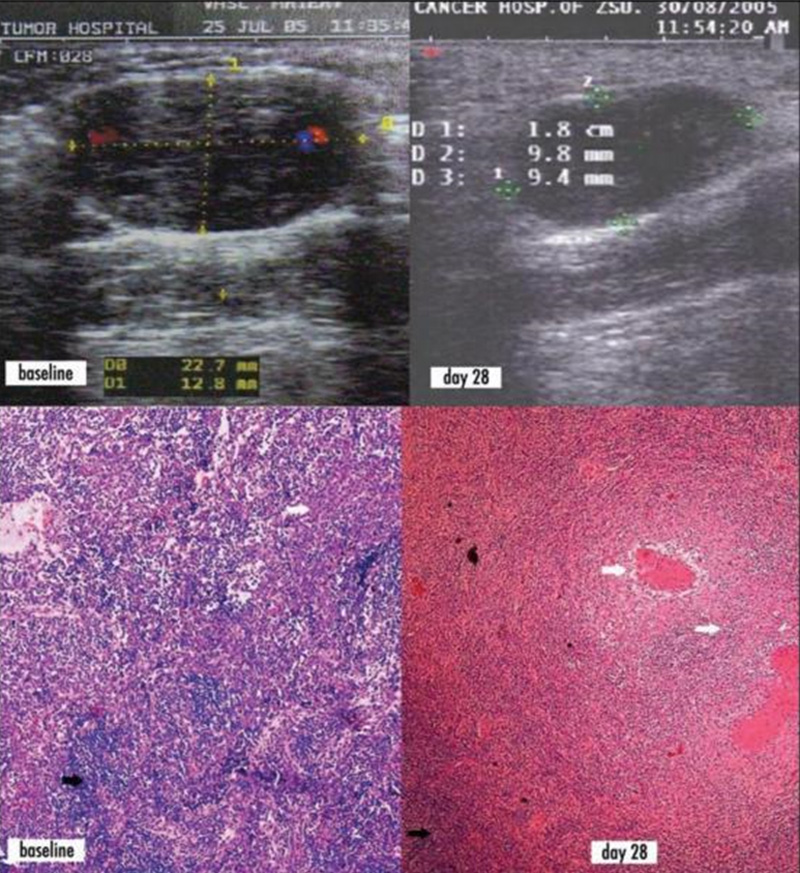
Case 3: Recurrence of nasopharyngeal carcinoma with submental lymph node metastasis. Ultrasound images showed that on day 28, the product of the longest diameter and the longest vertical diameter of the submental lymph node mass decreased from 22.7 mm × 12.8 mm at baseline to 18 mm × 9.8 mm. Baseline tumor biopsy: lymph nodes are replaced by tumor cells (HE staining, 4x microscope), white arrows indicate nasopharyngeal carcinoma cells, black arrows indicate residual lymphoid tissue; tumor biopsy on day 28: white arrows indicate tumor necrosis and fibrosis, black arrows indicate residual tumor tissue.
Phase III clinical study (E10A combined with TP chemotherapy) - case
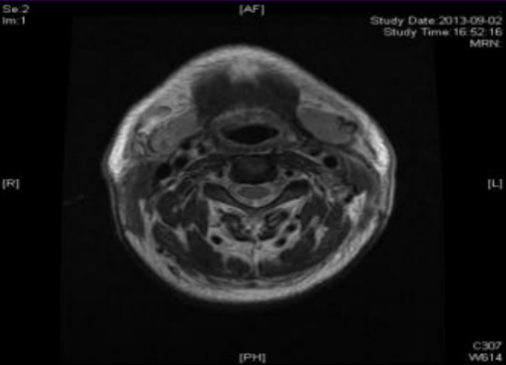
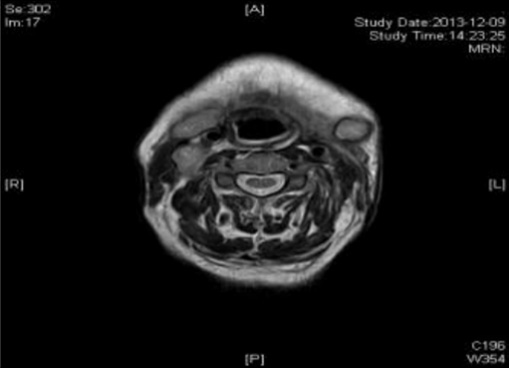
Case 1: Before the patient took the medicine, the size of the superficial lymph node in the 1-V area of the right neck was: 20mm*15mm; after two courses of treatment, the size was: 9mm*7mm
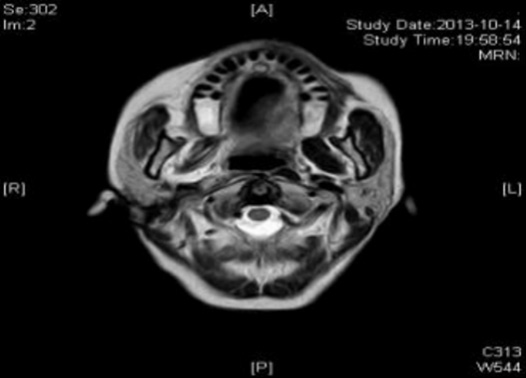
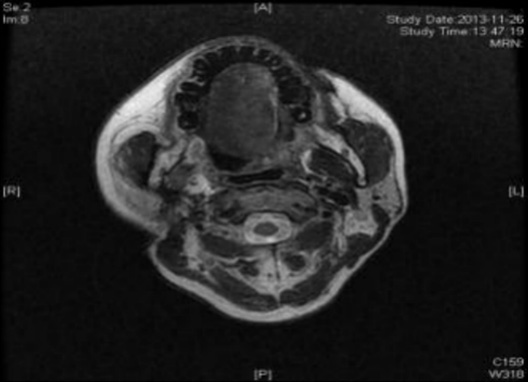
Case 2: Before the patient took the medicine, the size of the tumor under the right collar was: 49mm*61mm; after 4 courses of treatment, the size of the tumor was: 26mm*27mm
- None
- Previous






